Review and Approve GxP Documents with Egnyte
Multiple team members within a life science company may need to periodically review and approve GxP documents for compliance purposes, including those on the clinical, regulatory, quality and product teams. And with so many parties involved, you need to establish a clear and uniform set of instructions to ensure the process is a success.
However, if you manage the document review process by email, or need to switch from paper to electronic records for better retention—as many firms do today—it can be a very manual, time-consuming task, fraught with potential mistakes. These include:
- colleagues accidentally making changes to the wrong version
- missing due dates within the body of an email
- unauthorized users making changes to a file
While looking to minimize these errors, you may also be busy coordinating communication with reviewers on the draft or finalization status—leading to wasted time, extended review cycles, and other inefficiencies.
Why You Should Use Egnyte GxP for Review and Approval Workflows
With Egnyte, you can speed up the review and approval process via automated notifications and workflows. Egnyte GxP, a compliance portal that centralizes and supports auditing, validation, and reporting, provides an intuitive application to help reduce your administrative burden and the likelihood of errors.
You also have the flexibility to see draft versions of a document, co-edit in Microsoft 365 or Google Workspace, and assign role permissions for easy and secure access—for internal and external collaborators. In addition, teams can perform e-signatures for approvals in compliance with 21 CFR Part 11 and Annex 11. You can approve a range of documents types without the need for third-party plugins, including:
- Standard operating procedures (SOPs)
- Informed consent forms (ICFs)
- Quality processes
- Regulated documents such as validation docs
- Clinical trial protocols
How to Streamline and Speed Up the Review Cycle With Egnyte GxP
Streamlining the review and approval process is easy with Egnyte. After you create a draft document in the Egnyte GxP folder (or one of its subfolders), you can start completing and approving a review and approval workflow for any file type, such as a Microsoft 365 document, a Google Doc, or other productivity application file formats.
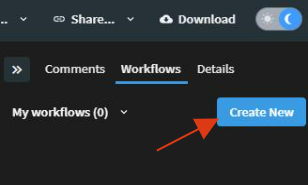
Create a Workflow
First, you need to create a workflow. From the file preview, under the Workflows menu, choose a one-step task, or a multi-step workflow for each stage of review, including final approval.
When you create a folder, you can easily grant and rescind access to files for internal and external collaborators. You can also set granular permissions, including limits on who can view, download, edit, or move files. This way, only users with assigned permission can modify records, ensuring no unauthorized changes can’t be made to effective documentation.
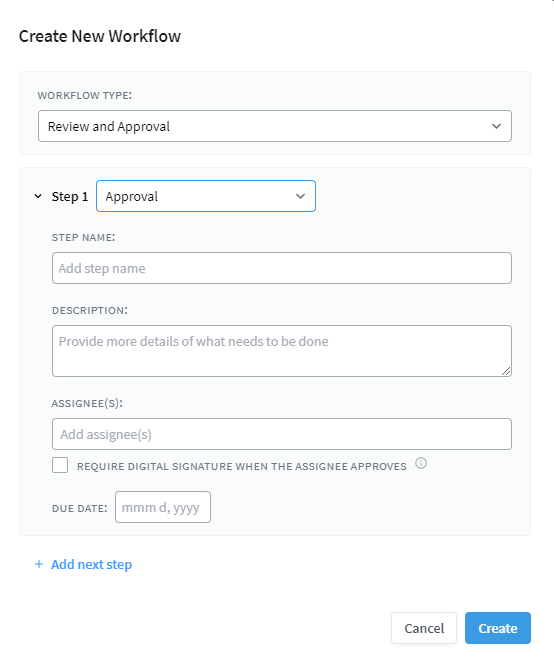
Choose Assignees and Set Due Dates
Next, choose who the workflow will be assigned to, set due dates, and click Create. You can also define version numbers for new drafts, and set expiration dates for document review or retirement.
Assignees will be notified of new task assignments via email and can also see all of their assignments in Tasks assigned to me.
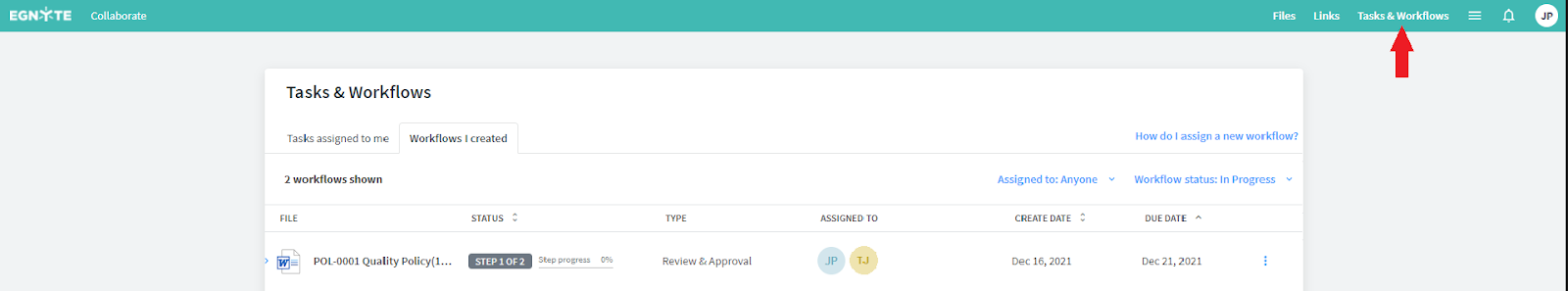
Access Your Tasks and Workflows
Whether you created the workflow or are an assignee, you also have visibility into the document status by navigating to the Tasks & Workflows tab in the upper right corner of the page.
Obtain Digital Signatures
Finally, in the GxP domain, approval includes Part 11-compliant digital signatures. The download will be a PDF rendition that includes a signature page at the end. If a digital signature is required for approval, a signature screen will be shown asking you to enter your username, password, and a reason for approving the document.
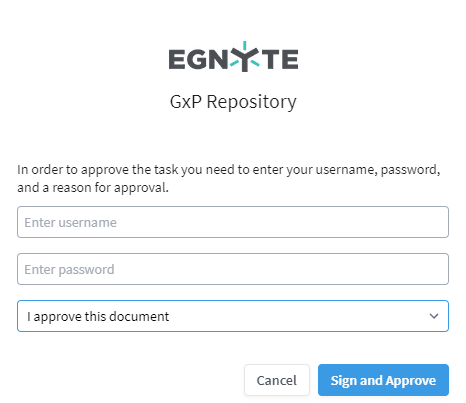
The system stores a cryptographic digital signature to validate the integrity of the signature. Workflows and digital signature records are permanently retained and cannot be deleted. This is an important capability for life sciences companies, which often need to ensure the integrity and secure retention of documents as determined by regulatory requirements. All actions taken as part of a workflow are tracked in the Workflow audit report.
And that's it. In just a few quick steps, you have ensured your review and approval process will go smoothly and securely.
To learn more, check out all the ways Egnyte can help your business with Egnyte for Life Sciences.





