Why Companies Don't Archive (Common Challenges and Pitfalls in TMF Archival)
Failure to archive a completed Trial Master File (TMF) is, to put it simply, non-compliant. Despite this fact, pharmaceutical and biotech companies are often known to drag their feet on this process. Why would such organizations expose themselves to that risk? Unfortunately, there are several common hurdles that teams face when it comes to TMF archival. In this post, we’ll walk through what those hurdles are and how to overcome them.
It’s too complicated
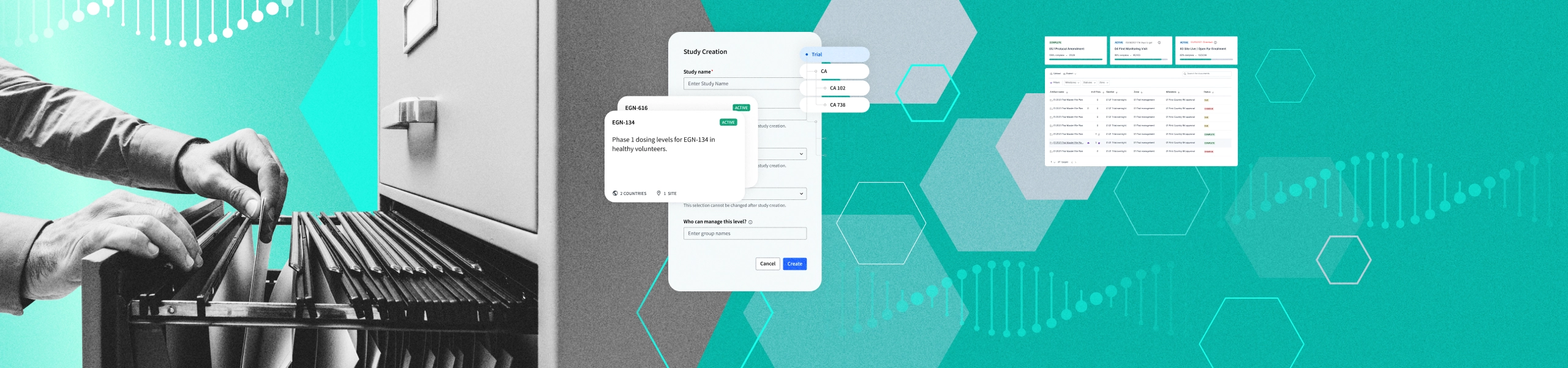
When archiving a study, many changes need to occur. A huge amount of data and documents need to be moved, and permissions need to change. Without automated tools in place, making all of those manual changes is painfully time-consuming and can also be complicated and confusing.
A good archival solution should automate all of the heavy lifting. Ideally, the feature should be as close as possible to a one-click archival solution.
It’s too expensive
Many organizations hesitate to store archived studies in an eTMF system due to associated costs. If the solution isn’t perceived as user-friendly or effective, it is simply “not worth it” compared to an in-house solution.
This is especially difficult to navigate when using a system where in-progress TMFs and archived TMFs come with different price tags. If imported TMFs aren’t auto-archived, this can increase the bill and feel unfair.
Generally, organizations should look to purchase an archival solution with reasonable and straightforward pricing, which allows them to import TMFs straight into the archive without paying extra fees.
It doesn’t work
After going through the work of archiving a study, users will be understandably unhappy if the end result isn’t fully compliant. Subpar archival solutions are either not protected enough, with too many users having free access to the content, or are too locked down, making it difficult to edit archived documents that require changes or to add content to the archived study. They may also lack a formal Archivist role, relying on other roles to fit the need.
All of these are potential issues— regulations state that content must be protected from unauthorized changes and managed by a designated Archivist. The right TMF solution should approach archival features accordingly.
The TMF isn’t ready
We all know how tough it can be to step away from something and call it “done.” Folks can feel the same way about a TMF!
In theory, a study should be archived when it’s complete, and all documents are accounted for. However, it’s very common for documents to “turn up” long after everything should have already been turned in.
If an archival solution isn’t sufficiently flexible to allow for easy addition of documents to an already-archived study, customers may put off archiving until much later than is recommended.
A strong TMF archival offering should allow document addition to archived studies in a manner that is simple and compliant.
Archival made easy
Do these challenges sound familiar? If archival woes are causing you headaches, we invite you to explore Egnyte’s fully compliant, simple, and straightforward eTMF archival solution. Click here now to learn more!