Life Sciences Solution
Collaboration With CROs, Sites and Partners
The easiest way for distributed teams to access, share and collaborate on documents from anywhere, on any device
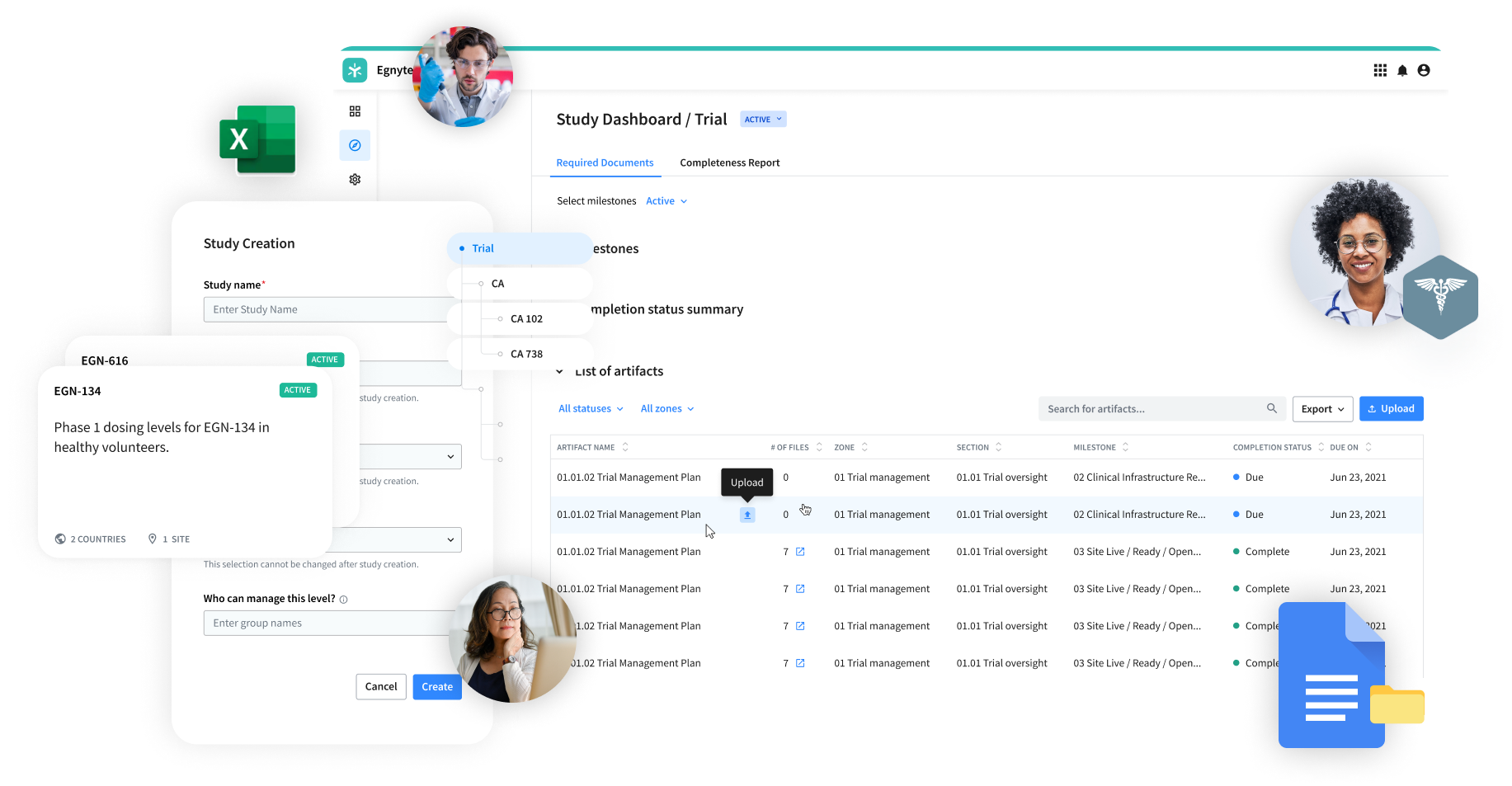
Common Challenges Collaborating With CROs
Access
- Frustrating remote access with VPN
- Lack of support for mobile devices
Performance
- File size limitations when sending data via ftp and email
- Lots of file duplication
- Limited ability to co-edit while maintaining version control
Security
- Unsanctioned, unmonitored sharing methods
- Limited ability to expire or revoke file access
- No visibility into file and folder access and user permissions
The Egnyte Solution
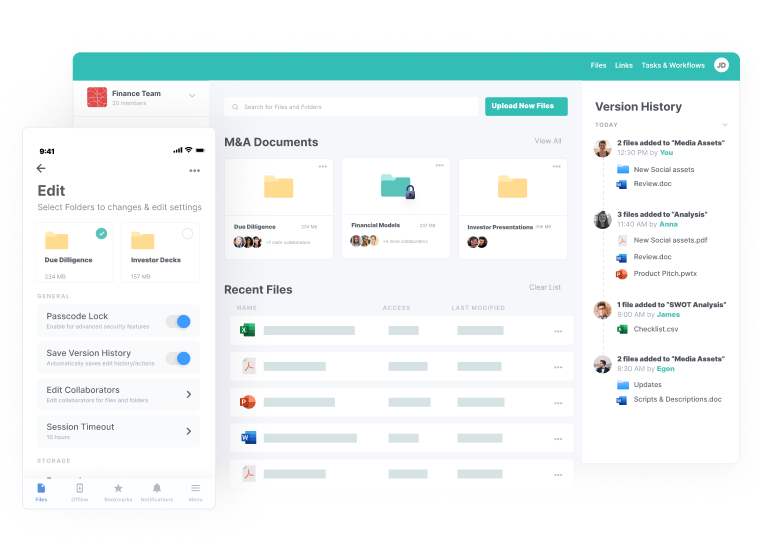
Centralized Access
- File access from anywhere, on any device
- Reliable availability in the cloud
- Built-in granular security and user controls
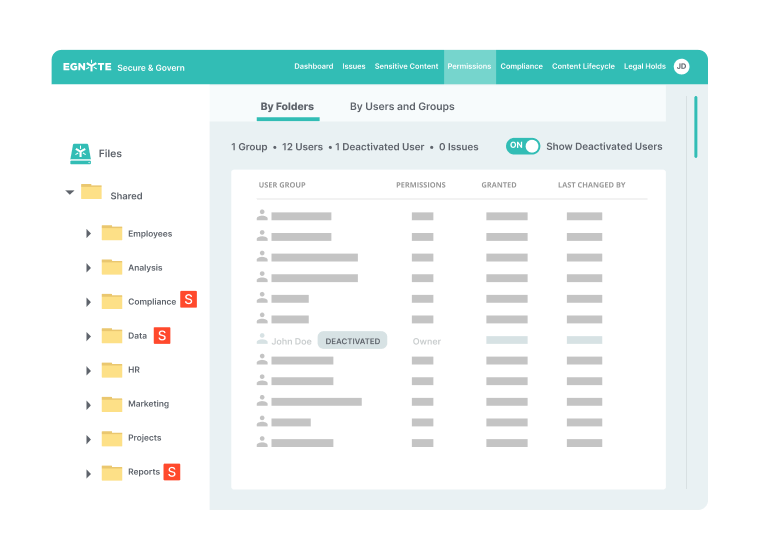
Performance for Distributed Teams
- File access and synchronization while working offline
- File visibility and management regardless of user location
- Access via native tablet and mobile apps
- Automated file uploads from multiple partners
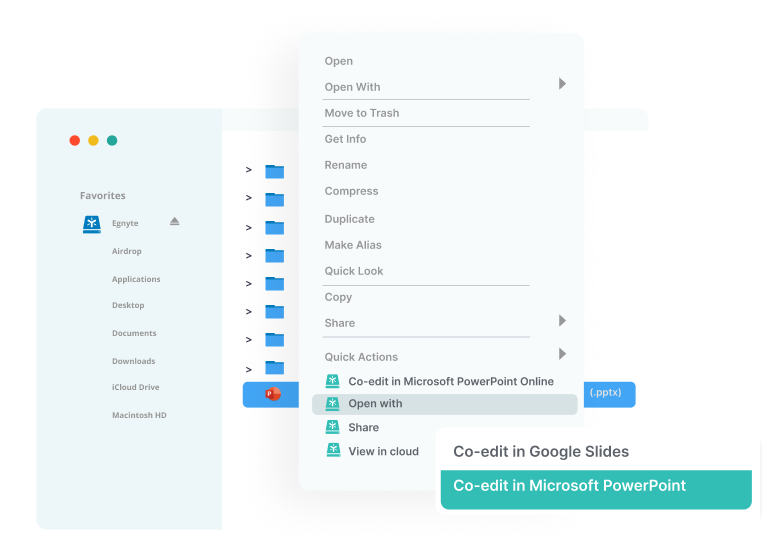
Seamless Collaboration
- Familiar 'drive letter' experience
- Search and access files within common industry applications
- Real-time co-editing in M365 and Google apps
- Preview-only file and folder links
- Built-in review and approval document workflows
Egnyte Works the Way You Do

Making Life Easier for Sponsors, CROs, Sites and Partners by Connecting to the Data and Tools that Matter the Most.
Easiest Way to Collaborate with CROs
The Power of Many Tools in One Turnkey Platform
Egnyte’s modular offerings grow with your company through all development and approval phases - from startup to scale up.
Egnyte
- Secure Collaboration with Partners
- Workflow Orchestration
- Authorized Access Controls
- Data Management
- Sensitive Data Discovery
- Document Lifecycle Management
Egnyte for Life Sciences
- GxP Compliance Portal
- Validation as a Service
- Life Science Specific Integrations
- eTMF Management and Archival
- Controlled Documentation and Training


