Controlled Document Management
Controlled Document Management for Emerging Biotech
Unifying controlled documentation processes, content, and training management onto a single cloud platform drives GxP compliance and operational excellence. Egnyte reduces the administrative burden of running an effective document management process by streamlining review and approval workflows, automating document status and providing comprehensive reporting capabilities.
Automate Processes
Control GxP-compliant documents with automated review and approval workflows.
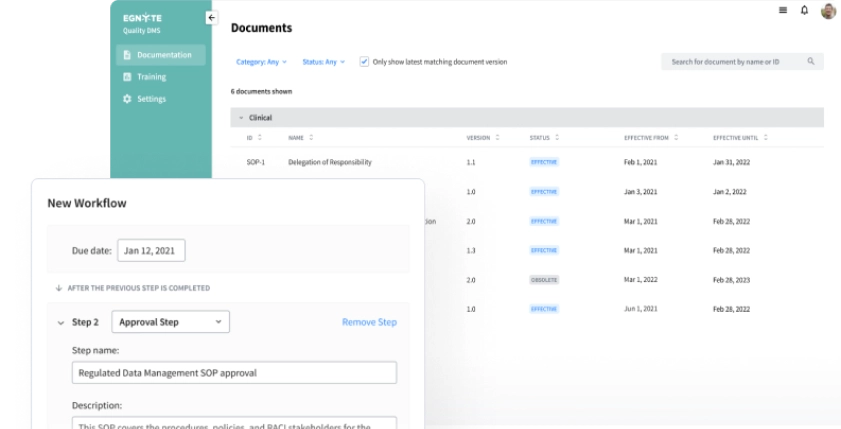
Control Documentation
Achieve greater visibility and control over regulated content and data across all GxPs.
Streamline Training
Increase training efficiency and compliance with an end-to-end solution.
Egnyte for Life Sciences Controlled Document Management
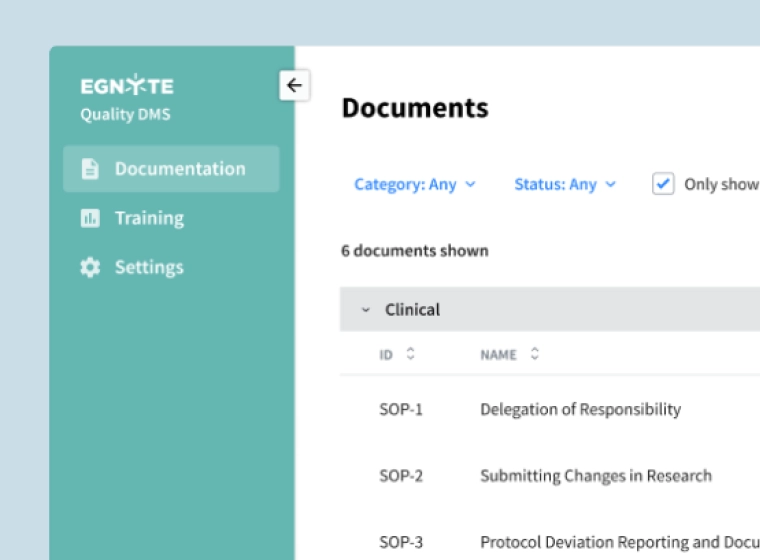
Smarter Document Management Processes
Streamline controlled document management, removing bottlenecks to regulated business processes.
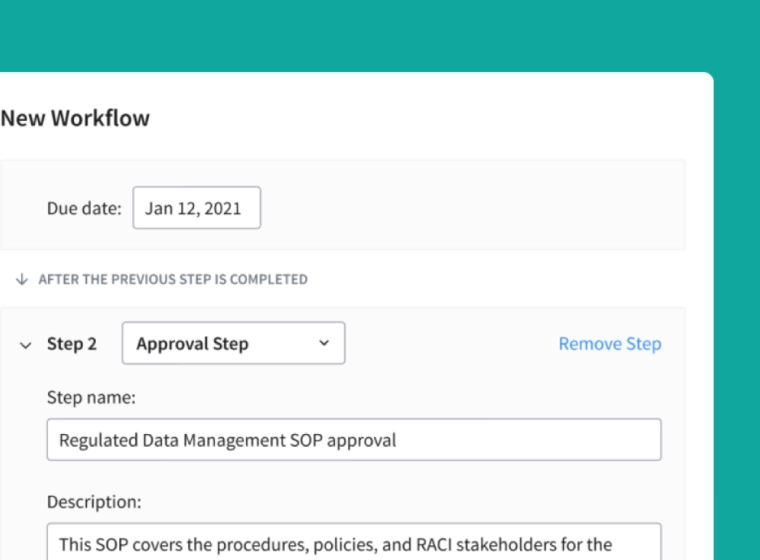
Meet FDA 21 CFR Part 11 Requirements
Accelerate the review and approval process with real-time cloud-based collaboration tools, notifications, and native Part 11-compliant e-signatures.
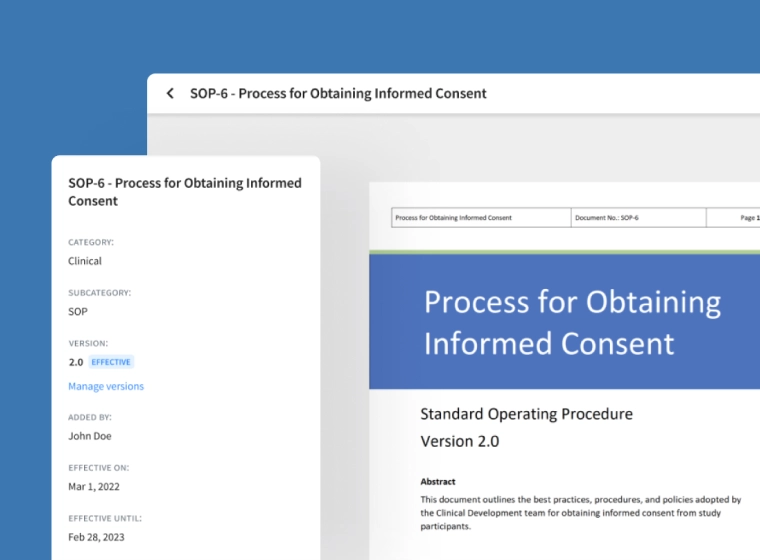
Manage Effective Documents Easily
Easily define version numbers for new documents, set expiry dates and ensure only effective copies are available.
Scale Compliance Processes Quickly
Integrate with existing IT infrastructure and quickly implement and validate the solution for rapid time to value.
The Power of Many Solutions In One Turnkey Platform
GxP
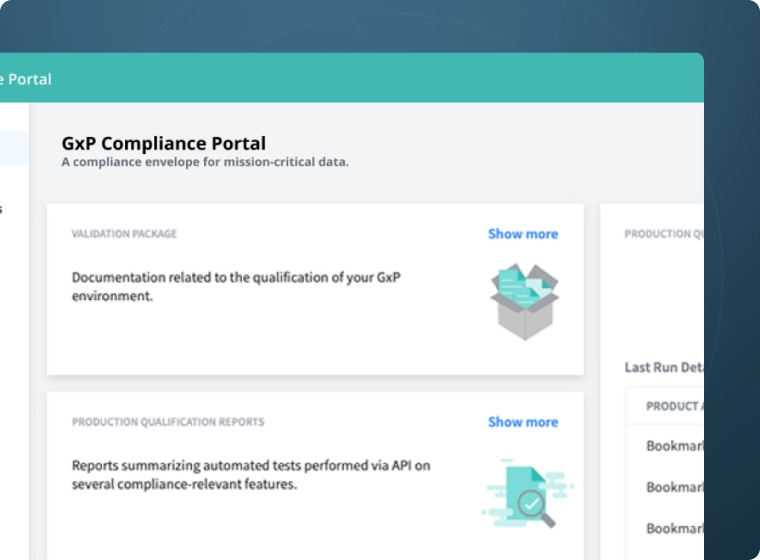
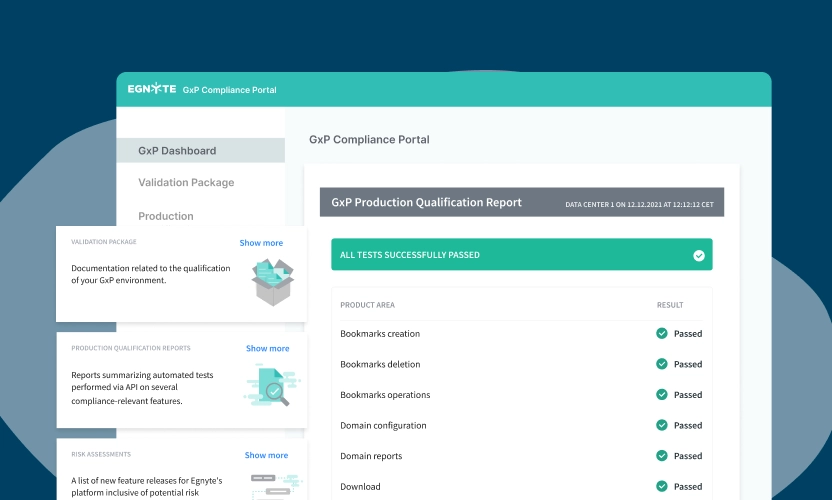
- GxP Compliance Portal
- Rapid Validation
- Audit Trails
- Multi-Step Workflows
- Data Lifecycle Management
Controlled Document Management
- Document Management
- Automated Review & Approval
- Training Module
Over 600 Life Sciences Customers Trust Egnyte
See Egnyte’s Document Management Solution in Action
See How Egnyte for Life Sciences Streamlines
Controlled Document Management
- Central location for effective documentation
- Review and approval workflows accelerate timeliness
- Date-based auto promotion of documents
